Inflammation: Obesity, Diabetes, Aging and Exercise
Common causes of chronic inflammation—and what you can do about it.
Inflammation is an essential defense system that has evolved in living organisms to enhance survival. The common triggers of inflammation are infection and tissue injury (Medzhitov 2008). Air pollution, poor water quality and other environmental factors can also trigger and sustain inflammation.
Medzhitov explains that the inflammatory response is the body’s attempt to restore itself to homeostasis—a state of equilibrium in which cellular parameters, such as glucose and oxygen, are within acceptable ranges. The blood is the delivery system for all anti-inflammatory agents. Inflammation involves macrophages (white blood cells that surround and kill microorganisms and remove dead cells), leukocytes (white blood cells that attack foreign substances and disease), and various other cellular components (Chung et al. 2019).
A first line of defense is short-term acute inflammation, in which immune cells, anti-inflammatory agents and tissue-remodeling processes act against tissue injury or risky substances (i.e., antigens such as allergens, toxins or pathogens) (Chung et al. 2019).
Chronic Inflammation and Chronic Disease
Acute inflammation is vital, but problems arise when the acute inflammatory response fails to resolve the harmful cellular intrusion. More defense components are mobilized, leading to a persistent, low-grade, long-term immune response known as chronic inflammation and a host of potentially serious health challenges (see “The Progression From Acute to Chronic Inflammation,” below).
Libby (2007) summarizes strong evidence that chronic inflammatory pathways contribute decisively to the development of a number of chronic diseases. This article will focus first on the association between chronic low-grade inflammation and obesity, type 2 diabetes and aging. Then it will review how exercise can combat chronic inflammation.

Obesity-Induced Inflammation
Most human beings live in countries where overweight and obesity kill more people than underweight does. In 2016, approximately 11% of men and 15% of women in the global adult population were considered obese. Woefully, 38 million of the world’s children under age 5 were obese or overweight in 2019 (WHO 2020a).
Clinically, overaccumulation of adipose tissue (fat) contributes to the development of hypertension, atherosclerosis, type 2 diabetes, hypercholesterolemia, nonalcoholic fatty liver disease, gall bladder disease, arthritis, Alzheimer’s disease and cancer (Ferrante 2007). White adipose tissue is no longer considered inert tissue committed solely to energy storage (Ellulu et al. 2017), and the relationship between obesity and inflammation has led to some fascinating research.
See also: The Food-Inflammation Connection
Fat and Inflammation: The Basics
Adipose tissue is composed of adipocytes (where fat is stored), pre-adipocytes, and various other types of immune cells (Boutens & Stienstra 2016).
Adipose tissue is stored in subcutaneous (below the skin) and visceral (around internal organs) storage depots. Epidemiological evidence indicates that visceral fat mass is a dominant risk factor for the development of metabolic abnormalities, including insulin resistance (Boutens & Stienstra 2016).
Adipocytes
It has been shown that, at the cellular level, obesity changes adipose tissue structure. Obesity leads to alterations in the composition and number of immune cells produced by adipocytes. The outcome is a low-grade inflammation that is associated with insulin resistance and type 2 diabetes (Boutens & Stienstra 2016).
As adipocytes continue to enlarge with obesity, blood supply to these fat cells decreases, causing hypoxia (low blood oxygen) and, consequently, endothelial dysfunction (Ellulu et al. 2017). Ellulu and colleagues show that endothelial dysfunction is dangerous because it increases the vasoconstriction response in large arteries responsible for supplying oxygenated blood to active tissue.
Adipokines and Cytokines
The immune cells in adipose tissue produce and secrete a wide variety of adipokines. Adipokines are a family of hormones—with both pro- and anti-inflammatory effects—that facilitate communication to other cells and tissues involved in satiety (feeling of fullness) and regulation of energy metabolism (Boutens & Stienstra 2016).
Interestingly, early studies revealed that people with obesity have higher circulating concentrations of systemic markers of inflammation compared with lean individuals.
As obesity develops, adipose tissue begins storing excess triglycerides, which can alter the release of adipokines and cytokines (small proteins that are crucial in controlling the growth and activity of other immune system cells). This imbalanced release leads to a systemic inflammatory process that interferes with insulin signaling.
As further explained by Boutens & Stienstra, experiments have shown that the inflammatory status, determined by the release of cytokines, is elevated in visceral fat as compared with subcutaneous fat.
Obesity has been found to increase the secretion of TNF-, an inflammatory cytokine that has an influence on whole-body lipid and glucose metabolism. Obesity may also increase the expression (i.e., the process by which the information encoded in a gene is used to direct the assembly of a protein molecule) of more than a dozen genes that produce inflammatory proteins (Ferrante 2007).
Additionally, overproduction of interleukin-6 (IL-6, a powerful pro-inflammatory cytokine) has been linked to decreases in adiponectin. Adiponectin is an important protein hormone known for reducing inflammation and arterial stiffness. Further, excess IL-6 stimulates cells along blood vessels to produce and secrete C-reactive protein. High levels of C-reactive protein are indicative of a chronic inflammatory state, infection and risk for coronary heart disease (Ellulu et al. 2017).
As with many factors in inflammation, balance is important, as you’ll see in the discussion of exercise and IL-6, below.
Macrophages
Macrophages, those white blood cells that maintain tissue homeostasis by clearing out cellular debris, play a key role in adipose tissue functioning and chronic inflammation. In humans who are lean, macrophages make up about 5% of the cells in adipose tissue. But in people with obesity, macrophages can constitute up to 50% of all adipose tissue cells (Boutens & Stienstra 2016), and they are continuously exposed to a surplus abundance of lipids.
This overloading of the macrophages leads to dysfunction in these cells, and the result is chronic inflammation of the adipose tissue (Ferrante 2007). Scientists are just beginning to understand this macrophage metabolism that occurs during obesity (Boutens & Stienstra 2016).
Inflammation, Type 2 Diabetes and Diabesity
The major health impacts of type 2 diabetes mellitus are long-term medical problems, including nephropathy (kidney disease), neuropathy (nerve damage), retinopathy, cardiovascular disease, peripheral vascular disease, stroke and periodontal abnormalities (Farag & Gaballa 2011).
From 1980 to 2014, the number of diabetes cases rose from 108 million to 422 million worldwide. Additionally, 1.6 million deaths internationally are attributed to diabetes each year (WHO 2020b). Further, some estimates reveal that the number of undiagnosed cases varies from 24.1% to as high as 75.1%, with an estimated 83.8% coming from low- and middle-income countries (Beagley et al. 2014).
As with obesity, the relationship between diabetes and inflammation is both complex and fascinating.
Diabetes
Researchers have found an association between low-grade inflammation and the development of type 2 diabetes (Schmidt & Duncan 2003). Chronic inflammation, characterized by an elevation of pro-inflammatory cytokines, can induce changes that lead to type 2 diabetes (Keane et al. 2015). Schmidt & Duncan observe that an elevated leukocyte count is the most consistently reported biomarker for diabetes. Leukocytes (white blood cells that attack foreign substances and diseases) are a major component of the body’s defenses against disease. Elevated levels of C-reactive protein are another inflammation marker consistently found to predict the onset of diabetes.
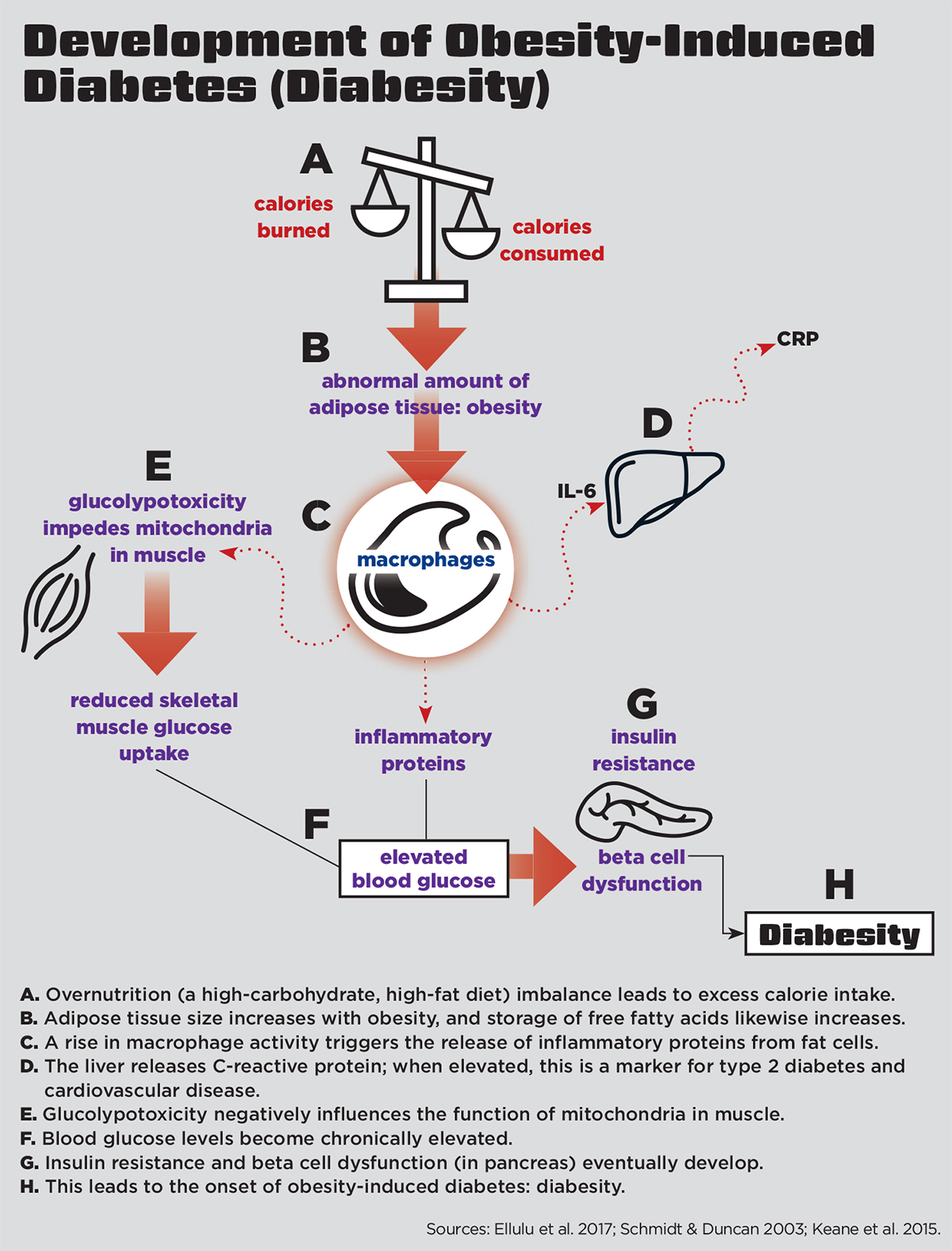
The mechanisms that exacerbate low-grade inflammation in diabesity are complex; however, the process begins primarily through overnutrition (a high-carbohydrate, high-fat diet) (Keane et al. 2015) (see “Development of Obesity-Induced Diabetes [Diabesity],” below).
Farag & Gaballa note that people with type 2 diabetes are more likely to develop various levels of depression, with research showing that the risk is up to three times higher than it is for people without diabetes. Knowing these data, Farag & Gaballa recommend screening people for depression in the early stages of a diabetes diagnosis.
Diabesity
Diabesity is a relatively new term in the scientific literature. In context, it means diabetes caused by or highly associated with obesity. Diabesity is sometimes referred to as obesity-dependent diabetes (Farag & Gaballa 2011).
In diabesity, chronic overnutrition induces a “glucolypotoxic” state (Keane et al. 2015). In other words, if a diet is constantly full of high carbohydrates (gluco) and fats (lypo), a toxic physiologic state may result. Glucolypotoxicity negatively influences the function of mitochondria (the energy factories of cells) (Keane et al. 2015). In a glucolypotoxic state, the energy-producing capacity of mitochondria becomes impaired, leading to a buildup of potentially damaging mitochondrial byproducts called reactive oxygen species molecules (also known as free radicals). Free radicals are unstable, highly reactive molecules that can damage RNA, DNA and proteins.
The buildup of free radicals stimulates low-grade inflammation throughout peripheral tissues in the body, as well as in beta cells (the cells that synthesize and secrete insulin) in the pancreas (Keane et al. 2015). Eventually, this leads to desensitization and blocking of the insulin signaling pathway and dysfunction of beta cells (Keane et al. 2015). Continual exposure to elevated glucose and lipids will impede the pancreatic beta cell secretion of insulin.
In a state of diabesity, the insulin signaling pathway becomes blocked by inflammatory cytokines inhibiting the muscle, fat and liver cells from using glucose. Skeletal-muscle mitochondrial cells are especially affected by this, as skeletal muscle is the principal tissue responsible for insulin-stimulated glucose disposal, accounting for up to 75% of glucose uptake (Zierath, Krook & Wallberg-Henriksson 2000). Eventually, insulin resistance and chronically elevated blood glucose levels will lead to type 2 diabetes.
Farag & Gaballa also cite research indicating that chronic stress may promote the development of diabesity through activation of inflammatory, autonomic, hormonal and immunological systems and suggest that targeting stress management should be a cornerstone in the treatment of diabesity.
See also: Weight Training and Diabetes
Inflammation and Aging
Aging is a process of adaptations over time controlled by numerous physiological and genetic factors associated with and/or responsible for the susceptibility to disease and eventual death. Among individuals, the rate of this process varies based on lifestyle, genetic predisposition and environmental factors that contribute to the inflammatory state.
Chronic low-grade inflammation is a contributor to various age-related pathologies, including hypertension, obesity, diabetes, atherosclerosis, cancer, etc. (Sanada et al. 2018). In addition, natural processes in aging tissue may lead to loss of mobility and function due to diseases such as sarcopenia (loss of muscle) and/or osteoporosis (loss of bone) (Sanada et al. 2018).
Free Radicals
Among the factors influencing the aging process is the production of free radicals (Liguori et al. 2018). As mentioned earlier, free radicals are highly reactive. That’s because they are molecules with an unpaired electron in the atomic orbital, a condition that causes instability. Free radicals are produced either from normal cell metabolism or from external sources, such as pollution, cigarette smoke, radiation or medication.
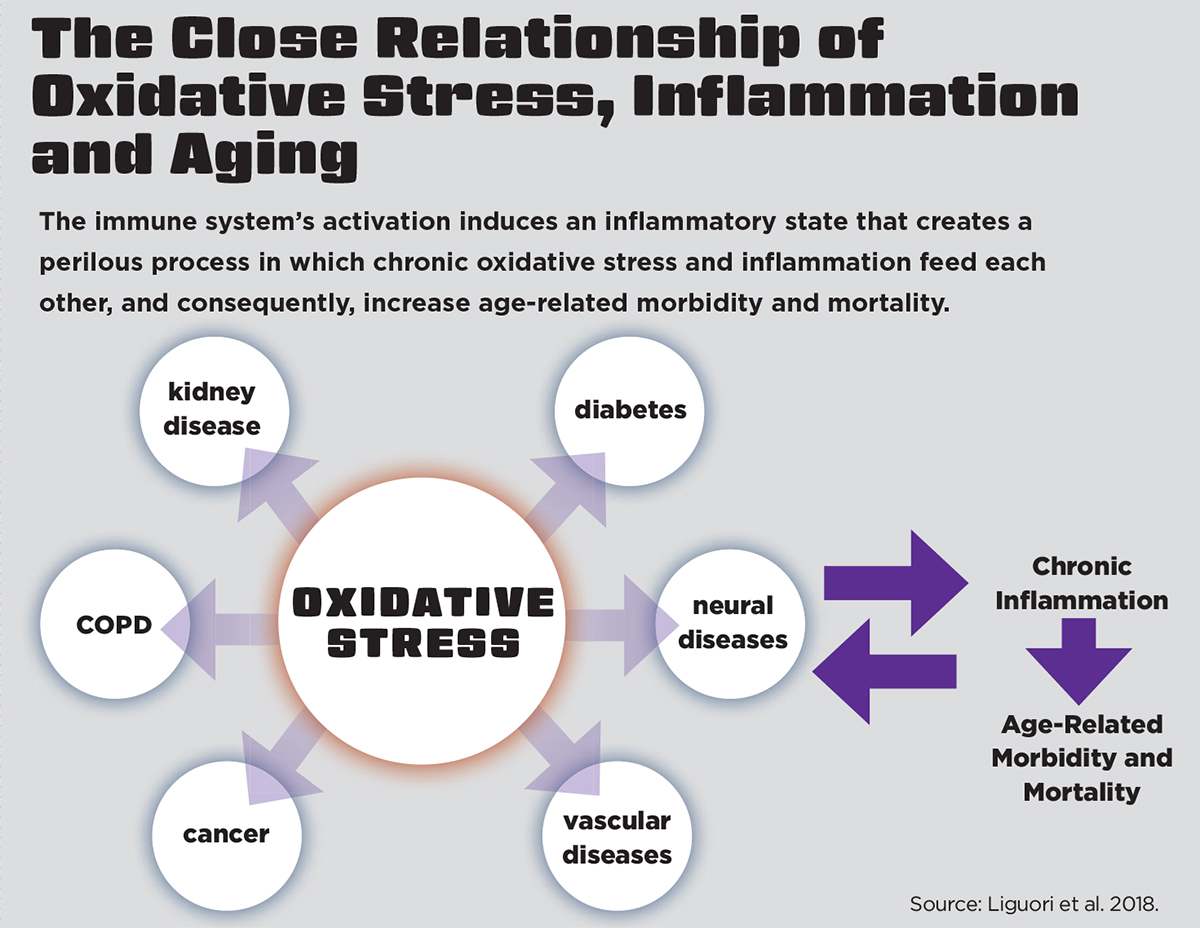
Pham-Huy, He & Pham-Huy (2008) explain that when an overload of free radicals cannot be neutralized, their accumulation in the body generates a phenomenon called oxidative stress (see “The Close Relationship of Oxidative Stress, Inflammation and Aging,” above). We become more susceptible to oxidative stress during the aging process as a result of this free-radical accumulation.
Oxidative stress eventually starts deteriorating target cells and organs in the body (Pham-Huy, He & Pham-Huy 2008). This stress leads to an inflammatory response through the activation of macrophages (white blood cells that surround and kill microorganisms and remove dead cells) in the immune system (Chung et al. 2019). Increased macrophage infiltration into almost all tissues—including liver, muscle, adipose tissue, brain, kidney and heart—is likely to trigger the pro-inflammatory process at the tissue level (Chung et al. 2019).
Oxi-Inflamm-Aging
Given the close relationship between oxidative stress, inflammation and aging, Liguori et al. (2018) have proposed the oxidation-inflammatory theory of aging, or “oxi-inflamm-aging.”
They explain that aging is a loss of homeostasis that occurs when regulatory systems, like the immune system, experience chronic oxidative stress. Activation of the immune system induces an inflammatory state that creates an undesirable cycle in which chronic oxidative stress and inflammation feed each other, increasing the risk for age-related morbidity and mortality. Fortunately, regular physical activity is a valid strategy for combating oxidative stress and the resultant age-related inflammatory disruptions (Liguori et al. 2018).
See also: Power Up Your Aging Clients
How Physical Activity Combats Inflammation
Regular physical activity is an effective protector and treatment against chronic diseases associated with chronic low-grade inflammation.
Skeletal muscle is the largest organ in the human body (Pedersen 2007). Muscle contractions from exercise increase the release of a specific type of cytokines, called myokines. The initial and primary cytokine associated with exercise is interleukin-6, which increases exponentially as a response to exercise (Mathur & Pedersen 2008).
With exercise, IL-6 acts as an energy sensor and begins to boost its production, potentially rising as much as a hundredfold compared with resting levels (Mathur & Pedersen 2008). The degree to which exercise raises circulating levels of IL-6 in the whole body correlates directly to the intensity, duration and amount of muscle mass recruited to perform the exercise (Pedersen 2007; Lee & Jun 2019).
Lee & Jun explain that IL-6 also stimulates glucose update and fatty-acid oxidation. Plus, the increase in IL-6 with exercise stimulates the use of fat as a fuel source for energy. The rise in IL-6 inhibits TNF-a production and ignites the release of anti-inflammatory cytokines into circulation (Pedersen 2007). Therefore, the rise in IL-6 caused by exercise may help counteract the role TNF-a plays in vascular inflammation, insulin resistance and type 2 diabetes (Pedersen 2007).
Aerobic Exercise
Nieman (2003) states that the most important finding that has emerged from exercise immunology research is that positive immune changes occur during each bout of moderate physical activity. He notes that this translates to fewer days of sickness with the common cold and other upper-respiratory-tract infections. When researchers compared the effects of low- versus moderate-intensity exercise, they found that moderate intensity was more successful in managing low-grade inflammation (Krause et al. 2014).
A study of male and female patients with stable coronary heart disease showed that the anti-inflammatory benefits of aerobic exercise can be achieved using a variety of modalities. For instance, researchers have found moderate-intensity aerobic exercise performed for 45 minutes 3 days a week for 12 weeks, on equipment such as a treadmill, stationary bike, arm bicycle, rowing machine or combination of these options, was effective at reducing basal levels of several pro-inflammatory proteins as well as increasing an anti-inflammatory cytokine (Goldhammer et al. 2005).
For previously sedentary individuals, the intensity of aerobic exercise can gradually progress from low to moderate over the course of months and can have a similar, very positive effect on low-grade inflammation (Kohut et al. 2006).
Higher-intensity exercise for a sustained period of time is not encouraged for immune system health. The literature shows that many components of the immune system show adverse change after prolonged, heavy exercise lasting longer than 90 minutes (Nieman 2003).
In regard to decreasing the production of free radicals and oxidative stress and increasing the levels of antioxidants (which neutralize free radicals), the evidence shows that for elderly individuals, endurance exercise at 50%–80% of VO2max on 2–3 days per week is optimal (Liguori et al. 2018).
Resistance Exercise
As an essential component of fitness, resistance training provides health-related benefits such as improvements in the metabolic profile of people with type 2 diabetes, increased muscle mass for slowed progression of sarcopenia, increased bone mineral density for prevention of osteoporosis and overall improvements in body composition (Calle & Fernandez 2010).
Resistance training consists of three muscle contractions: eccentric, isometric and concentric. The eccentric, or lengthening, phase often induces the greatest muscle damage. This tissue injury triggers an inflammatory response that stimulates the release of cytokines. From the cascade of white blood cells reaching the damaged tissue, free radicals are produced, causing further tissue damage and synthesis of more cytokines (Calle & Fernandez 2010). This can lead to muscle soreness and swelling but also, eventually, to tissue repair and remodeling (Calle & Fernandez 2010; Izquierdo et al. 2009). Additionally, the IL-6 produced by growing muscle fibers acts as a regulator for satellite cell–mediated muscle hypertrophy (Izquierdo et al. 2009).
Resistance exercise programming (intensity, volume and rest intervals) also influences the inflammatory response. A single bout raises the number of cytokines; however, adaptations from long-term training elicit a decrease in cytokines and a shift to an anti-inflammatory response (Calle & Fernandez 2010).
In most studies that have examined the effect of resistance training on inflammation, subjects have been older men and women. Liguori et al. summarize that resistance training improves antioxidant defenses, helping to neutralize free radicals and oxidative stress, in the elderly population.
This positive effect can be achieved when training protocols use moderate-intensity workloads and provide a sufficient volume of exercise (number of exercises, frequency and duration of intervention) for each muscle group (3–5 sets, 10 repetitions). Sardeli et al. (2018) concur that higher-volume workouts with moderate-intensity resistance training protocols appear to play a positive role in its anti-inflammatory effects.

Final Review: 8 Key Takeaways
- The common triggers of acute inflammation are infection and tissue injury.
- There is strong evidence that chronic inflammatory pathways contribute decisively to the development of a number of chronic diseases.
- Obesity leads to alterations in the composition and number of immune cells produced by adipocytes. The outcome is a low-grade inflammation that is associated with insulin resistance and type 2 diabetes. Inflammation is elevated in visceral fat as compared with subcutaneous fat.
- Chronic stress may promote the development of diabesity (obesity-induced diabetes) through activation of inflammatory, autonomic, hormonal and immunological systems.
- People with diabetes are over three times more likely to develop depression than those without diabetes.
- Muscle contractions from exercise increase the re–lease of a specific type of inflammatory proteins that help counteract insulin resistance and type 2 diabetes.
- Moderate-intensity aerobic exercise—relative to a person’s fitness level—is more effective in managing chronic low-grade inflammation as compared with low-intensity aerobic exercise. Strenuous exercise leads to greater oxidative stress and thus enhances the inflammatory responses of the body.
- A higher volume of resistance training protocols (more exercises, greater frequency and duration of intervention) appears to play a positive role in combating inflammation.
COVID-19 and Inflammation
Chronic inflammation is a factor in conditions such as diabetes, obesity and high blood pressure—all of which are seen in many people hospitalized with COVID-19. A striking facet of COVID-19 is that it has the capacity to cause so many different symptoms, further confusing the body’s immune system. Consequently, a viral infection that occurs during chronic inflammation puts the body’s immune system on continuous overload.
References
Beagley, J., et al. 2014. Global estimates of undiagnosed diabetes in adults. Diabetes Research and Clinical Practice, 103 (2), 150–60.
Boutens, L., & Stienstra, R. 2016. Adipose tissue macrophages: Going off track during obesity. Diabetologia, 59 (5), 879–94.
Calle, M.C., & Fernandez, M.L. 2010. Effects of resistance training on the inflammatory response. Nutrition Research and Practice, 4 (4), 259–69.
Chen, L., et al. 2018. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9 (6), 7204–18.
Chung, H.Y., et al. 2019. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging and Disease, 10 (2), 367–82.
Ellulu, M.S., et al. 2017. Obesity and inflammation: The linking mechanism and the complications. Archives of Medical Science, 13 (4), 851–63.
Farag, Y.M.K., & Gaballa, M.R. 2011. Diabesity: An overview of a rising epidemic. Nephrology Dialysis Transplantation, 26 (1), 28–35.
Ferrante, A.W., Jr. 2007. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. Journal of Internal Medicine, 262 (4), 408–14.
Goldhammer, E., et al. 2005. Exercise training modulates cytokines activity in coronary heart disease patients. International Journal of Cardiology, 100 (1), 93–99.
Izquierdo, M., et al. 2009. Cytokine and hormone responses to resistance training. European Journal of Applied Physiology, 107 (4), 397–409.
Keane, K.N., et al. 2015. Molecular events linking oxidative stress and inflammation to insulin resistance and b-cell dysfunction. Oxidative Medicine and Cellular Longevity, doi:10.1155/2015/181643.
Kohut, M.L., et al. 2006. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of b-blockers, BMI, and psychosocial factors in older adults. Brain, Behavior, and Immunity, 20 (3), 201–9.
Krause, M., et al. 2014. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: Implications for oxidative stress, low-grade inflammation and nitric oxide production. European Journal of Applied Physiology, 114 (2), 251–60.
Lee, J.H., & Jun, H.S. 2019. Role of myokines in regulating skeletal muscle mass and function. Frontiers in Physiology, 10, 42.
Libby, P. 2007. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutrition Reviews, 65 (12), S140–46.
Liguori, I., et al. 2018. Oxidative stress, aging, and disease. Clinical Interventions in Aging, 13, 757–72.
Mathur, N., & Pedersen, B.K. 2008. Exercise as a mean to control low-grade systemic inflammation. Mediators of Inflammation, doi:10.1155/2008/109502.
Medzhitov, R. 2008. Origin and physiological roles of inflammation. Nature, 454, 428–35.
Nieman, D.C., 2003. Current perspective on exercise immunology. Current Sports Medicine Reports, 2 (5), 239–42.
Pedersen, B.K. 2007. State of the art reviews: Health benefits related to exercise in patients with chronic low-grade systemic inflammation. American Journal of Lifestyle Medicine, 1 (4), 289–98.
Pham-Huy, L.A., He, H., & Pham-Huy, C. 2008. Free radicals, antioxidants in disease and health. International Journal of Biomedical Science, 4 (2), 89–96.
Sanada, F., et al. 2018. Source of chronic inflammation in aging. Frontiers in Cardiovascular Medicine, 5 (12).
Sardeli, A.V., et al. 2018. Effect of resistance training on inflammatory markers of older adults: A meta-analysis. Experimental Gerontology, 111, 188–96.
Schmidt, M.I., & Duncan, B.B. 2003. Diabesity: An inflammatory metabolic condition. Clinical Chemistrry and Laboratory Medicine, 41 (9), 1120–30.
WHO (World Health Organization). 2020a. Obesity and overweight. Accessed Oct. 24, 2020:
who.int/news-room/fact-sheets/detail/obesity-and-overweight.
WHO. 2020b. Diabetes. Accessed Nov. 7, 2020: who.int/news-room/fact-sheets/detail/diabetes.
Zierath, J.R., Krook, A., & Wallberg-Henriksson, H. 2000. Insulin action and insulin resistance in human skeletal muscle. Diabetologia, 43 (7), 821–35.
Len Kravitz, PhD
Len Kravitz, PhD is a professor and program coordinator of exercise science at the University of New Mexico where he recently received the Presidential Award of Distinction and the Outstanding Teacher of the Year award. In addition to being a 2016 inductee into the National Fitness Hall of Fame, Dr. Kravitz was awarded the Fitness Educator of the Year by the American Council on Exercise. Just recently, ACSM honored him with writing the 'Paper of the Year' for the ACSM Health and Fitness Journal.
Gabriella Bellissimo, MA
Gabriella Bellissimo, MA, ACSM-CES, ACSM-CPT, is a doctoral student in exercise science at the University of New Mexico in Albuquerque, where she works as a teaching assistant in the Human Performance Lab. Her research interests include investigating time-efficient and convenient forms of exercise to promote adherence and reduce the risk of metabolic and chronic diseases.
Jessica Smith, MS, CSCS
Jessica Smith, MS, CSCS, is a doctoral student in exercise science at the University of New Mexico in Albuquerque, where she works as a teaching and research assistant in the Health, Exercise and Sports Science Department. Her research interests include biomechanical and metabolic adaptations to resistance training and methodologies of program design.